HIGHLIGHTS
Perfecting the shot
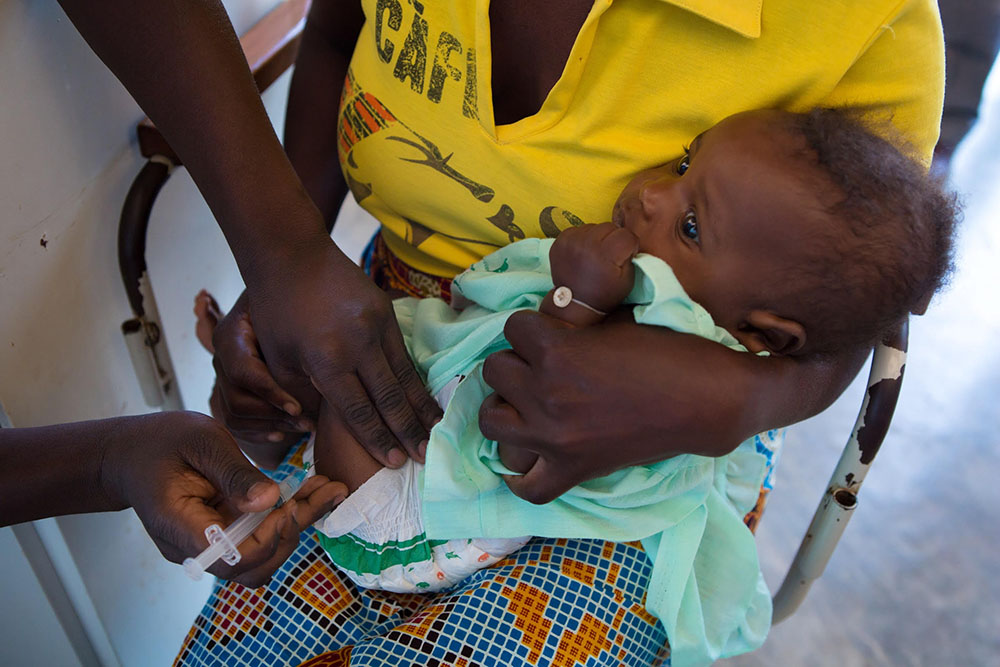
A series of papers provides further insights on the kind of immune response that can protect against malaria
Throughout human history, malaria may have killed half of the people who ever lived
Developing a vaccine against the malaria parasite—or any parasite, for that matter—has proven exceedingly challenging. Most vaccines contain inactivated pathogens or pathogen fragments against which the body produces protective antibodies – ideally, a complete, long-lasting protection. This is not the case for RTS,S (Mosquirix), which has the merit of being the first vaccine approved for large-scale pilot studies in Africa, but that protects only partially and for a limited amount of time. Over the last few years, ISGlobal researcher Carlota Dobaño and her group have tried to understand why the vaccine protects some children, but not others.
Quantity and quality
The team looked at the antibody response in over 1,000 infants (between 6 and 12 weeks of age) and children (5 to 17 months old) vaccinated or not during the RTS,S phase 3 clinical trial. They found that the protection conferred by the vaccine is not only a matter of antibody quantity, but also of quality: the more strongly some antibodies bind to their ligand (particularly to one end of the CSP parasite protein contained in the vaccine), the better protection they provide. The results also indicate that when a child has already been exposed to malaria (and therefore has anti-CSP antibodies), the protective effect of the vaccine is lower. “This suggests that the vaccine will better protect children who have been less exposed to the parasite, for example those living in low-transmission areas,” says Dobaño.
Broader protection
Interestingly, Dobaño’s team also found that the RTS,S vaccine (which includes only a fragment of the CSP protein) can induce protective antibodies against other parasite proteins not included in the vaccine. “We think that the partial efficacy of the vaccine allows low infection levels upon subsequent parasite exposure, which in turn leads to the production of protective antibodies,” explains Gemma Moncunill, who coordinated the study. “This effect would be observed especially in regions with low to moderate transmission levels,” she adds. Importantly, these results identify antigens that could be included in more effective multivalent vaccines in the future.

Photo: GAVI, The Vaccine Alliance.
Dobaño C, Sanz H, Sorgho H et al. Concentration and avidity of antibodies to different cirumsporozoite epitopes correlate with RTS,S/ASO1E malaria vaccine efficacy. Nature Communications. 2019 May 15;10(2174).
Dobaño C, Ubillos I, Jairoce C, et al. RTS,S/AS01E immunization increases antibody responses to vaccine unrelated Plasmodium falciparum antigens associated with protection against clinical malaria in African children: a case-control study. BMC Medicine. 2019 Aug 14;17(157).
HIGHLIGHTS
Perfecting the shot
A series of papers provides further insights on the kind of immune response that can protect against malaria
Photo: GAVI, The Vaccine Alliance.

Developing a vaccine against the malaria parasite—or any parasite, for that matter—has proven exceedingly challenging. Most vaccines contain inactivated pathogens or pathogen fragments against which the body produces protective antibodies – ideally, a complete, long-lasting protection. This is not the case for RTS,S (Mosquirix), which has the merit of being the first vaccine approved for large-scale pilot studies in Africa, but that protects only partially and for a limited amount of time. Over the last few years, ISGlobal researcher Carlota Dobaño and her group have tried to understand why the vaccine protects some children, but not others.
Quantity and quality
The team looked at the antibody response in over 1,000 infants (between 6 and 12 weeks of age) and children (5 to 17 months old) vaccinated or not during the RTS,S phase 3 clinical trial. They found that the protection conferred by the vaccine is not only a matter of antibody quantity, but also of quality: the more strongly some antibodies bind to their ligand (particularly to one end of the CSP parasite protein contained in the vaccine), the better protection they provide. The results also indicate that when a child has already been exposed to malaria (and therefore has anti-CSP antibodies), the protective effect of the vaccine is lower. “This suggests that the vaccine will better protect children who have been less exposed to the parasite, for example those living in low-transmission areas,” says Dobaño.
Broader protection
Interestingly, Dobaño’s team also found that the RTS,S vaccine (which includes only a fragment of the CSP protein) can induce protective antibodies against other parasite proteins not included in the vaccine. “We think that the partial efficacy of the vaccine allows low infection levels upon subsequent parasite exposure, which in turn leads to the production of protective antibodies,” explains Gemma Moncunill, who coordinated the study. “This effect would be observed especially in regions with low to moderate transmission levels,” she adds. Importantly, these results identify antigens that could be included in more effective multivalent vaccines in the future.
Dobaño C, Sanz H, Sorgho H et al. Concentration and avidity of antibodies to different cirumsporozoite epitopes correlate with RTS,S/ASO1E malaria vaccine efficacy. Nature Communications. 2019 May 15;10(2174).
Dobaño C, Ubillos I, Jairoce C, et al. RTS,S/AS01E immunization increases antibody responses to vaccine unrelated Plasmodium falciparum antigens associated with protection against clinical malaria in African children: a case-control study. BMC Medicine. 2019 Aug 14;17(157).